Impurity Isolation and Profiling
Planta Analytica provides advanced impurity isolation and profiling services tailored for pharmaceutical and agrochemical applications. Our analytical capabilities support regulatory compliance, product safety, and batch-to-batch consistency. Establishing a comprehensive impurity profile enables manufacturers to maintain rigorous production control and ensure batch-to-batch consistency. Additionally, isolated impurities play a vital role in assessing product safety by facilitating the evaluation of individual component toxicity.
The initial phase of impurity isolation involves a comprehensive analysis of the product to identify and prioritize impurities for targeted isolation. Utilizing high-precision analytical techniques ensures accurate identification of all critical components, optimizing resource allocation while maintaining compliance with product certification requirements
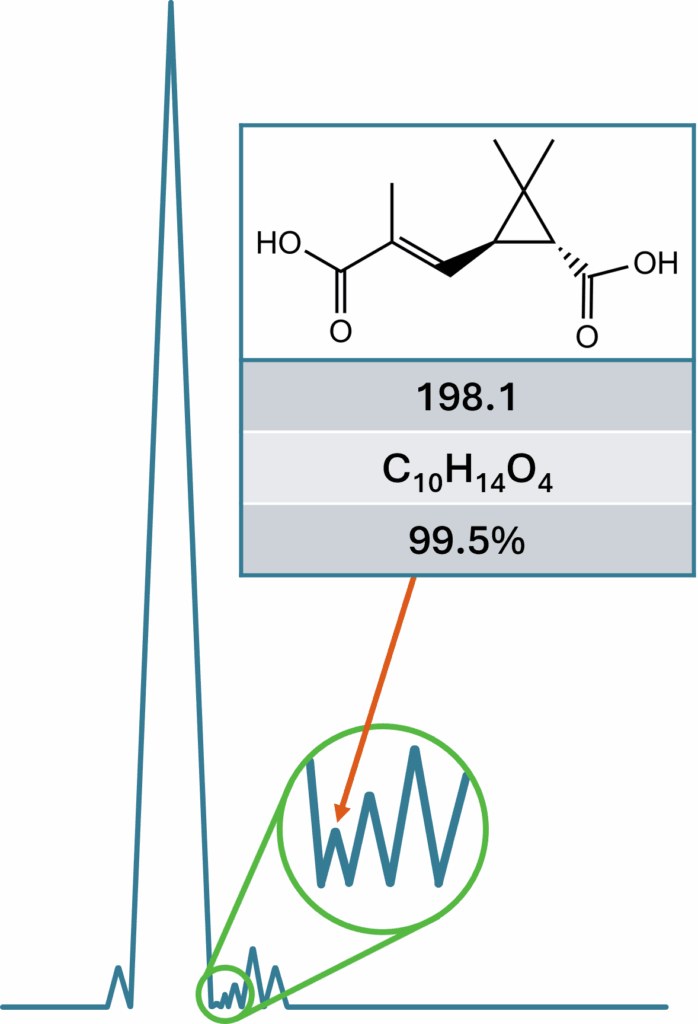
Generating high-quality data for applications such as toxicological studies and batch analysis requires the use of high-purity isolates as a foundation. With nearly two decades of expertise in addressing complex impurity isolation challenges, we have the capability to tackle even the most demanding isolation requests. For product characterization and certification, we collaborate with leading laboratories to obtain high-precision data through advanced analytical techniques, including NMR, mass spectrometry, UV-Vis spectroscopy, and any additional methods required. Our highly experienced team of structural elucidation and characterization chemists ensures the definitive identification of compounds with absolute confidence.
Impurity Profiling Highlights
As regulatory requirements continue to evolve, the documentation and certification process for products has become increasingly complex.
Since 2006, Planta Analytica has been a trusted partner for agrochemical companies, assisting with product registration and ensuring compliance with industry standards. Our expertise in regulatory documentation can help streamline your submission process, minimizing delays and facilitating smooth market certification
Sensitivity Analysis for Trace-Level Targets
Development of high-resolution analytical methods to determine with certainty which impurities exceed the minimum threshold as set by the regulatory agency.
Structural Elucidation
Expert-tier data interpretation and structural elucidation services for accurate characterization of your impurities. Comprehensive analysis for certification using HPLC-UV, ELSD, LC-MS, NMR, Residual Solvents, non-combustible solids analysis and more.
Semi & Fully Synthetic Production of Compounds
Isolation is not always the best path to produce certain impurity and fate targets. For these cases we offer semi synthesis and full synthesis services to produce your target compounds.
Comprehensive Reporting & Documentation
Certificates of Analysis are backed up with high-quality and comprehensive analytics and report that satisfy the stringent requirements of regulatory agencies.
How is it different from normal compound isolation?
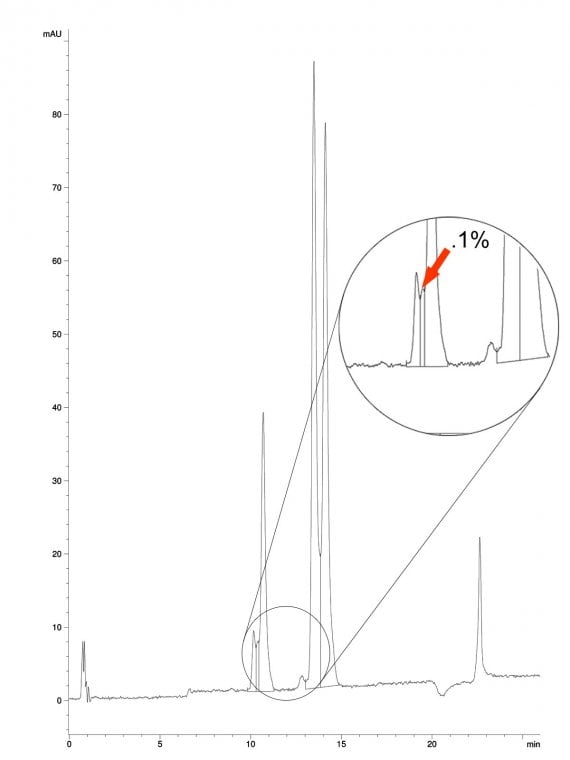
Impurity isolation, while categorized under small molecule isolation, presents distinct challenges due to the exceptionally low starting concentrations of target compounds—typically ranging between 0.05% and 0.5%. At these levels, conventional chromatographic techniques are ineffective, requiring a 10- to 50-fold concentration increase before preparative chromatography becomes economically feasible. Achieving this necessitates at least three distinct enrichment stages. These early-phase processes are often labor-intensive and complex, involving large sample volumes and masses relative to the minute quantities of the targeted impurities.
Further complications arise when isolating impurities from bacterially derived products, such as agrochemicals including avermectins, emamectins, and spinosyns. These substances typically consist of a single major component accompanied by multiple structurally similar impurities. The combination of structural resemblance and extreme concentration disparity significantly increases the difficulty of resolving and enriching the impurities.
Our Analytical Capabilities
Analytical Method Development for Quantitative Impurity Profiling
Our analytical method development services deliver regulatory-compliant protocols covering HPLC, TLC, colorimetric assays, and LC-MS validated for accuracy, precision, linearity, and sensitivity to meet FDA, EMA and ICH standards.
Production Batch Analysis & Certification
Ensure consistent active content and impurity control across every lot with our five-batch routine analysis and stability-indicating assays. We generate detailed Certificates of Analysis for R&D through full-scale production runs and integrate customized reporting dashboards to streamline your QC workflows.
Custom Method Deployment
Whether you require trace-level impurity quantification, uniformity testing or rapid screening, we tailor sample preparation and detection workflows to your matrix. From method transfer to derivatization optimization, we provide turnkey solutions that deliver high-precision data on time and within budget.
Ready to accelerate your impurity isolation and QC processes? Contact Planta Analytica today for a project consultation and personalized quote.


